产品详情
The schematic diagram of Laminin 511 in ES/iPS cell culture.
产品描述(Product Details)
Laminin 511 E8 (LN511-E8) is a recombinant human protein that provides a defined surface for the in vitro feeder-free culture of multiple human pluripotent stem cells (PSCs). As a truncated form of laminin 511, LN511-E8 is a functionally minimal form that retains the full capability for binding to integrins. LN511-E8 has been proven to maintain normal growth characteristics and stemness in multiple PSC lines without simultaneous differentiation, which includes ESC, iPSC, MSC, etc. In addition, LN511-E8 has been demonstrated to support PSC growth for >10 passages without any signs of karyotypic abnormalities and to maintain the ability of PSCs to differentiate into all three germ line lineages. As published by Takamichi Miyazaki et al., the LN511-E8 variant of laminin 511 shows higher efficiency for supporting the adhesion of dissociated cells than did wild-type laminin 511 which makes it a cost-effective choice.
High-quality products and regulatory support files are essential for the smooth transition from preclinical research & development to cell therapy clinical study. Designed for clinical research, GMP Laminin 511 is manufactured by an animal-free process in a GMP-compliant facility. A full battery of QC testing is implemented to ensure product quality, including purity, bioactivity, sterility, mycoplasma, endotoxin, etc. GMP Laminin 511 protein (GMP-LA1H25) is the GMP version of laminin 511 protein premium grade (LA8-H5283L), and they have the same performance for a seamless transition.
Optimized and defined surface
Laminin 511 E8 (LN511-E8) has been proven to allow single-cell seeding at low density. In a feeder-free culture system, the seeded cells demonstrate high motility with higher clonal survival.
Physiology-related
The laminin 511 isoform is crucial to the growth and maintenance of hPSCs in human through its binding to cell receptors a6B1 integrin. LN511-E8 is truncated laminin 511 isoform which works in multiple stem cell lines, from iPSC to hESC and MSC.
Better Adhesion
LN511-E8 variant of laminin 511 shows higher efficiency for supporting the adhesion of dissociated cells than did wild-type laminin 511.
Enhance cell differentiation
Due to the diverse biorelevant environment, GMP Laminin 511 could also enhance cell differentiation, polarization and organization of target cell types, including retinal pigmented epithelial cells, neurons, hepatocytes, cardiomyocytes, pancreatic β-cells, and so on.
Reduce Variability
LN511-E8 is a defined, recombinant human protein with better lot-to-lot consistency that reduces variability in your PSC cultures.优势特色(Features)
- Designed under ISO 9001:2015 and ISO 13485:2016
- Manufactured and QC tested under a GMP compliance factory
- Animal-Free materials
- Beta-lactam materials free
- Batch-to-batch consistency
- Stringent quality control tests
产品参数(Key parameter)
Purity (SDS PAGE)> 95%Mycoplasma TestNegativeSterility TestNegativeIntegrin Binding Assay1.0 nM < KD < 15 nMEndotoxin Test<10 EU/mgHost Cell Protein<0.5 ng/µgHost Cell DNA<0.02 ng/μg制剂(Formulation)
Supplied as 0.2 μm filtered solution in PBS, pH7.4 with protectants.
Contact us for customized product form or formulation.
运输(Shipping)
This product is supplied and shipped with dry ice, please inquire the shipping cost.
存储(Storage)
For long term storage, the product should be stored at liquid state at -70°C.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- 2-8°C for 6 months under sterile condition;
- -20°C or below for 3 years.
质量管理控制体系(QMS)
数据展示
活性(Bioactivity)-Stem Cell Culture
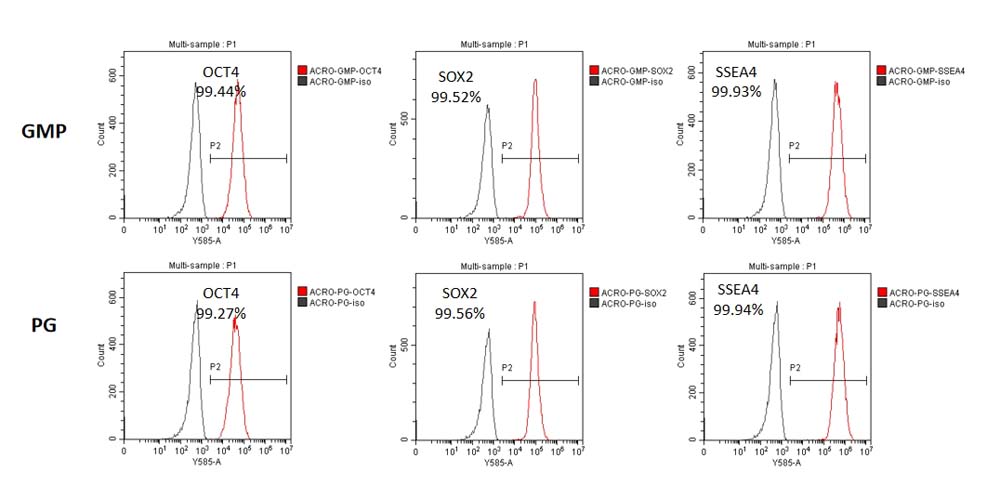
GMP Human Laminin 511 Protein (Cat. No. GMP-LA1H25) and Human Laminin 511 Protein, premium grade (Cat. No. LA8-H5283L) could maintain the stemness of iPSC at least Passage 5 and has similar performance. FACS data indicated that the iPSCs expressed high levels of pluripotency associated markers OCT4, SOX2, and SSEA4.
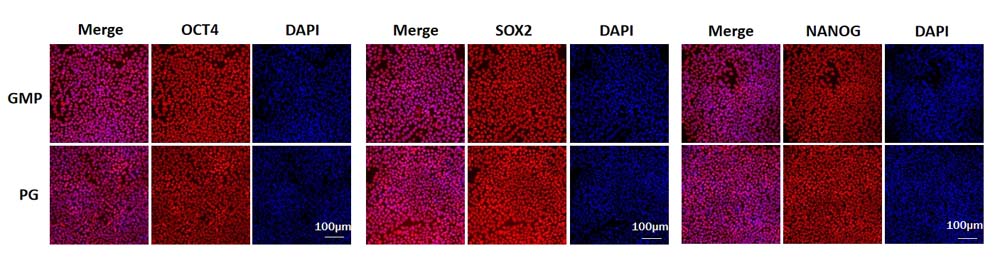
GMP Human Laminin 511 Protein (Cat. No. GMP-LA1H25) and Human Laminin 511 Protein, premium grade (Cat. No. LA8-H5283L) could maintain the stemness of iPSC at least Passage 5 and have similar performance. Immunofluorescence staining indicated that the iPSCs expressed high levels of pluripotency associated markers OCT4, SOX2, and NANOG.
活性(Bioactivity)-SPR
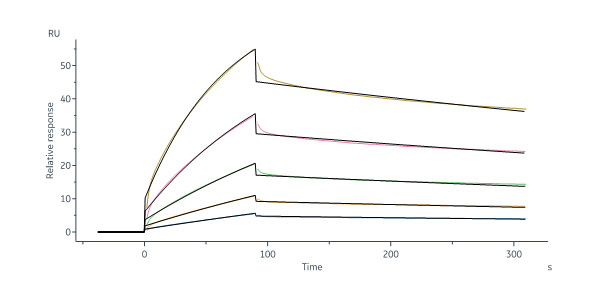
GMP Human Laminin 511 Protein (Cat. No. GMP-LA1H25) immobilized on CM5 Chip can bind Human Integrin alpha 6 beta 1 (ITGA6&ITGB1) Heterodimer Protein, His Tag&Tag Free with an affinity constant between 1.00 nM - 15.0 nM as determined in a SPR assay (Biacore 8K) (QC tested).
Protocol
用户评价 发表评论

背景介绍
High-quality products and regulatory support files are essential for the smooth transition from preclinical research & development to cell therapy clinical study. Designed for clinical research, GMP Laminin 511 is manufactured by an animal-free process in a GMP-compliant facility. A full battery of QC testing is implemented to ensure product quality, including purity, bioactivity, sterility, mycoplasma, endotoxin, etc. GMP Laminin 511 protein (GMP-LA1H25) is the GMP version of laminin 511 protein premium grade (LA8-H5283L), and they have the same performance for a seamless transition.
Optimized and defined surface
Laminin 511 E8 (LN511-E8) has been proven to allow single-cell seeding at low density. In a feeder-free culture system, the seeded cells demonstrate high motility with higher clonal survival.
Physiology-related
The laminin 511 isoform is crucial to the growth and maintenance of hPSCs in human through its binding to cell receptors a6B1 integrin. LN511-E8 is truncated laminin 511 isoform which works in multiple stem cell lines, from iPSC to hESC and MSC.
Better Adhesion
LN511-E8 variant of laminin 511 shows higher efficiency for supporting the adhesion of dissociated cells than did wild-type laminin 511.
Enhance cell differentiation
Due to the diverse biorelevant environment, GMP Laminin 511 could also enhance cell differentiation, polarization and organization of target cell types, including retinal pigmented epithelial cells, neurons, hepatocytes, cardiomyocytes, pancreatic β-cells, and so on.
Reduce Variability
LN511-E8 is a defined, recombinant human protein with better lot-to-lot consistency that reduces variability in your PSC cultures.
重要声明
MANUFACTURING SPECIFICATIONS
ACROBiosystems GMP grade products are produced under a quality management system and in compliance with relevant guidelines: Ph. Eur General Chapter 5.2.12 Raw materials of biological origin for the production of cell-based and gene therapy medicinal products; USP<92>Growth Factors and Cytokines Used in Cell Therapy Manufacturing; USP<1043>Ancillary Materials for Cell, Gene, and Tissue-Engineered Products; ISO/TS 20399-1:2018, Biotechnology - Ancillary Materials Present During the Production of Cellular Therapeutic Products.
ACROBiosystems Quality Management System Contents:
- Designed under ISO 9001:2015 and ISO 13485:2016, Manufactured and QC tested under a GMP compliance factory.
- Animal-Free materials
- Materials purchased from the approved suppliers by QA
- ISO 5 clean rooms and automatic filling equipment
- Qualified personnel
- Quality-related documents review and approve by QA
- Fully batch production and control records
- Equipment maintenance and calibration
- Validation of analytical procedures
- Stability studies conducted
- Comprehensive regulatory support files
Request For DMFACROBiosystems provide rigorous quality control tests (fully validated equipment, processes and test methods) on our GMP grade products to ensure that they meet stringent standards in terms of purity, safety, activity and inter-batch stability, and each bulk QC lot mainly contains the following specific information:
- SDS-PAGE
- Protein content
- Endotoxin level
- Residual Host Cell DNA content
- Residual Host Cell Protein content
- Biological activity analysis
- Microbial testing
- Mycoplasma testing
- In vitro virus assay
- Batch-to-batch consistency
DISCLAIMER
ACROBiosystems GMP grade products are designed for research, manufacturing use or ex vivo use. CAUTION: Not intended for direct human use.
TERMS AND CONDITIONS
All products are warranted to meet ACROBiosystems Inc.’s (“ACRO”) published specifications when used under normal laboratory conditions.
ACRO DOES NOT MAKE ANY OTHER WARRANTY OR REPRESENTATION WHATSOEVER, WHETHER EXPRESS OR IMPLIED, WITH RESPECT TO ITS PRODUCTS. IN PARTICULAR, ACRO DOES NOT MAKE ANY WARRANTY OF SUITABILITY, NONINFRINGEMENT, MERCHANTABILITY OR FITNESS FOR ANY PARTICULAR PURPOSE.
NOT WITH STANDING ANY OTHER PROVISIONS OF THESE TERMS AND/OR ANY OTHER AGREEMENT BETWEEN ACRO AND PURCHASER FOR THE PURCHASE OF THE PRODUCTS, ACRO’S TOTAL LIABILITY TO PURCHASER ARISING FROM OR IN RELATION TO THESE TERMS, AN AGREEMENT BETWEEN THE PARTIES OR THE PRODUCTS, WHETHER ARISING IN CONTRACT, TORT OR OTHERWISE SHALL BE LIMITED TO THE TOTAL AMOUNT PAID BY PURCHASER TO ACRO FOR THE RELEVANT PRODUCTS. IN NO EVENT WILL ACRO BE LIABLE FOR THE COST OF PROCUREMENT OF SUBSTITUTE GOODS.
END USER TERMS OF USE OF PRODUCT
The following terms are offered to you upon your acceptance of these End User Terms of Use of Product. By using this product, you indicate your acknowledgment and agreement to these End User Terms of Use of Product. If you do not agree to be bound by and comply with all of the provisions of these End User Terms of Use of Product, you should contact your supplier of the product and make arrangements to return the product.
The End User is aware that ACROBiosystems Inc. and its affiliate (“ACRO”) sell GMP grade products designed for research, manufacturing use or ex vivo use and not intended for human in vivo applications. The End User further agrees, as a condition of the sales of ACRO’s GMP grade products that: a) the End User will not use this GMP grade product in any procedure wherein the product may be directly or indirectly administered to humans, unless the End User has obtained, or prior to their use will have obtained, an Investigational New Drug (IND) exemption from the FDA and will use the product only in accordance with the protocols of such IND and of the Institutional Review Board overseeing the proposed research, or b) the End User will use the products outside of the United States in accordance with the protocols of research approved by the applicable review board or authorized ethics committee and regulatory agencies to which the End User is subject to in their territory.