产品详情
背景(Background)
PD-L1 is an informative immunotherapy biomarker: Aberrant expression of PD-L1 on tumor and immune cells in the tumor microenvironment impedes anti-tumor immunity, allowing tumors to grow and metastasize. The PD-L1 IHC 5D3 Kit is a quick assay to detect PD-L1 across tumors to identify patients eligible for multiple targeted therapies. Kit product are manufactured in a dedicated GMP facility and are compliant with our ISO 13485 quality management system.
产品描述(Product Details)
AnalytePD-L1Format7.5mlReactivityHumanSpeciesRabbitRegulatory StatusRUOApplicable PlatformLEICA BOND III、DAKO link 48组分(Materials Provided)
Recombinant Monoclonal Anti-PD-L1 Antibody, Rabbit (5D3)
Antibody diluent
应用说明(Application)
The kit is developed for use in the PD-L1 protein in the formalin-fixed, paraffin-embedded tissue. It is indicated as an aid in the assessment of PD-L1 expression in human tissues.
状态(State)
Liquid
存储(Storage)
Stored at 2°C to 8°C.
质量管理控制体系(QMS)
数据展示
质控样本(Control Sample)
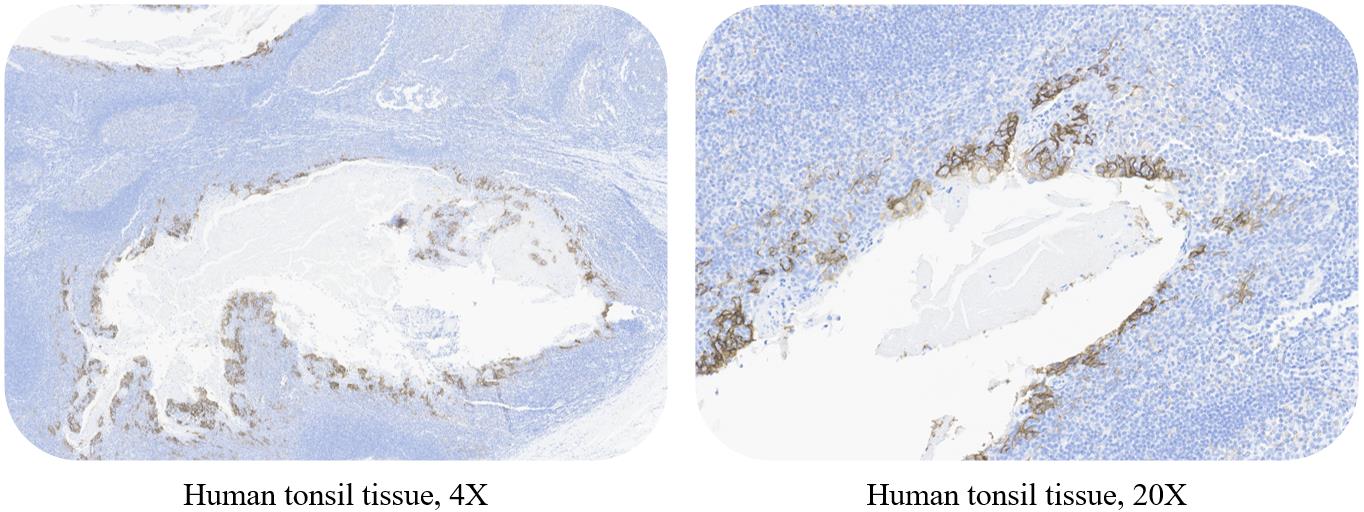
Use ACRO PD-L1 5D3 kit to stain human tonsil tissue, positive cell membrane specific positive staining (staining intensity ≥ 1), no non-specific staining (staining intensity < 1) , no background staining (staining intensity < 1 ).
一致性(Consistency)
ACRO PD-L1 5D3 and DAKO PD-L1 22C3 conformance verification, with a high consistency rate (>95%).
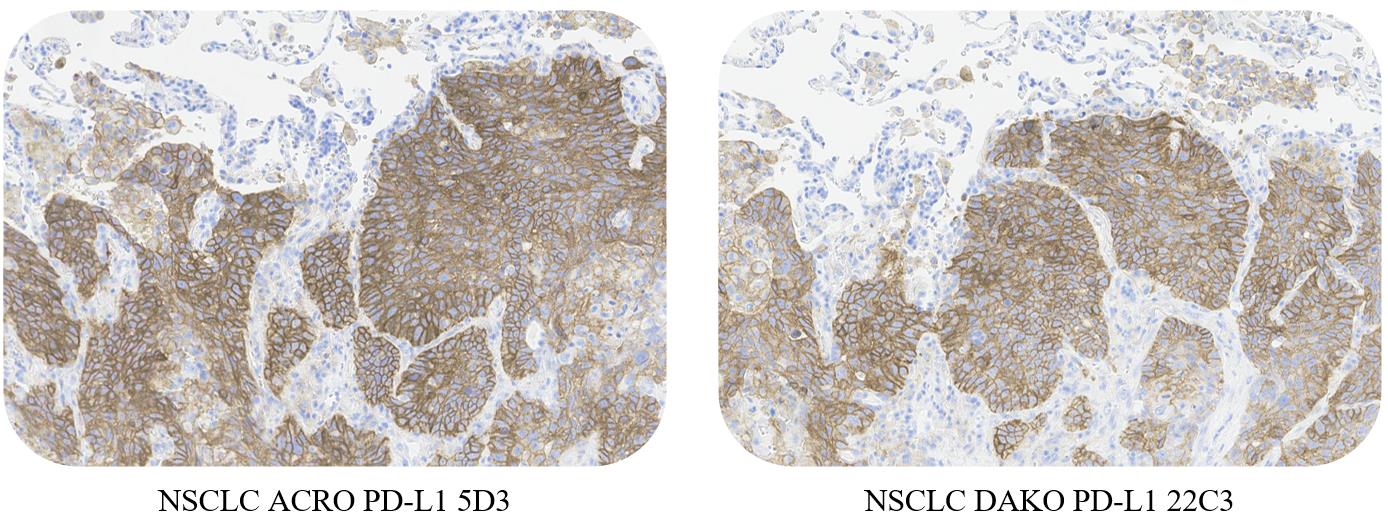
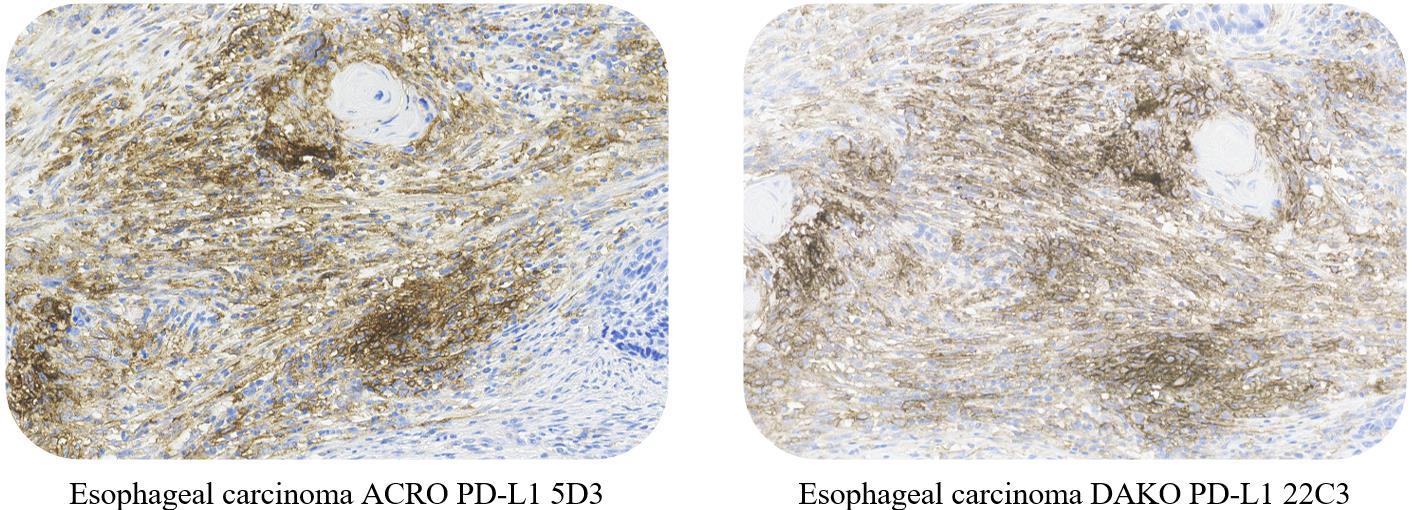
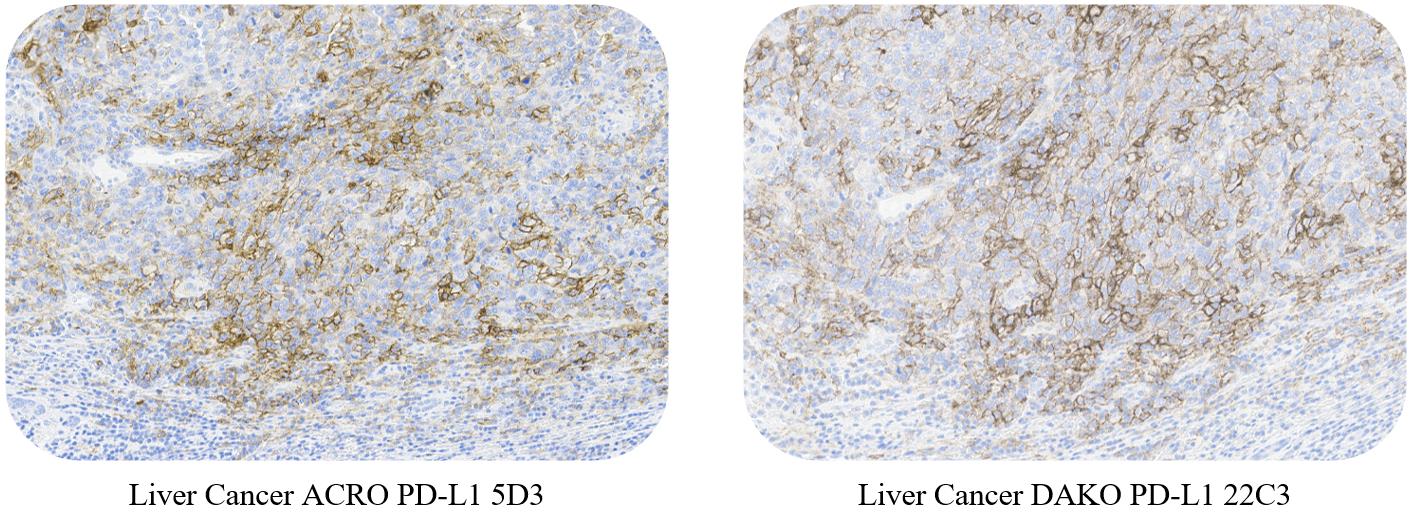
Use ACRO PD-L1 5D3 Kit and DAKO PD-L1 22C3 kit to stain non-small cell lung cancer, esophageal squamous cell carcinoma and liver cancer, respectively. The results showed that the staining effect was highly consistent.
扩展性(Extensibility)
ACRO PD-L1 5D3 kit expandable test: non-small cell lung cancer, esophageal carcinoma, gastric cancer, colorectal cancer and liver cancer.
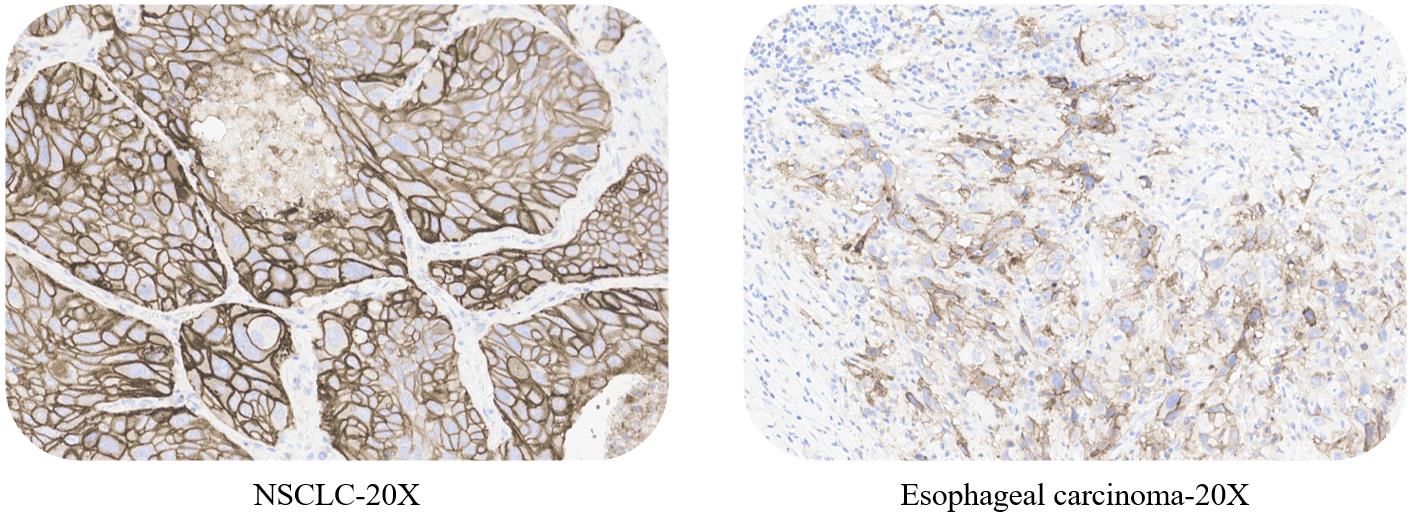
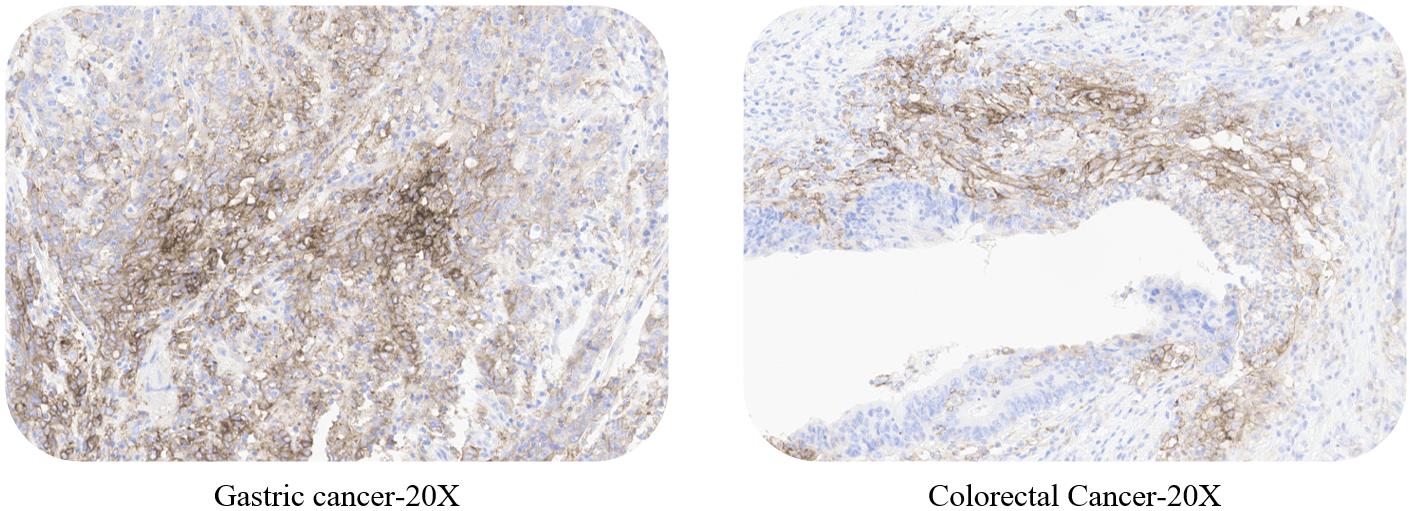
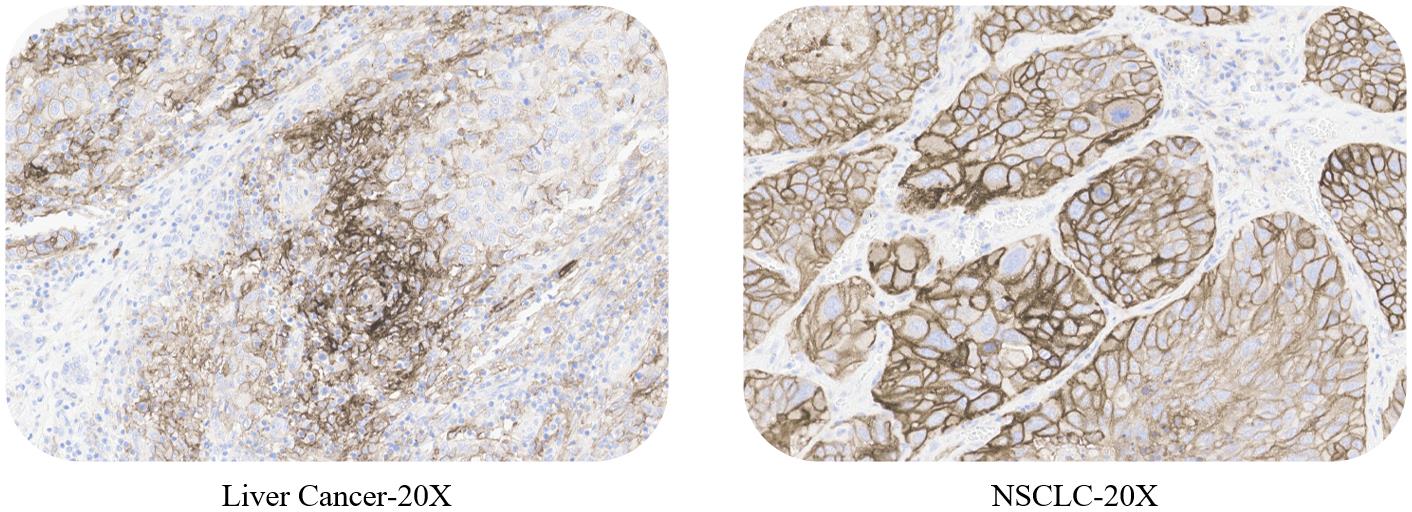
Use ACRO PD-L1 5D3 kit to stain NSCLC, esophageal carcinoma, gastric cancer, colorectal cancer, liver cancer tissue samples respectively.
适用性(Applicability)
ACRO PD-L1 5D3 can be available on the LEICA BOND III and DAKO Link 48 platform.
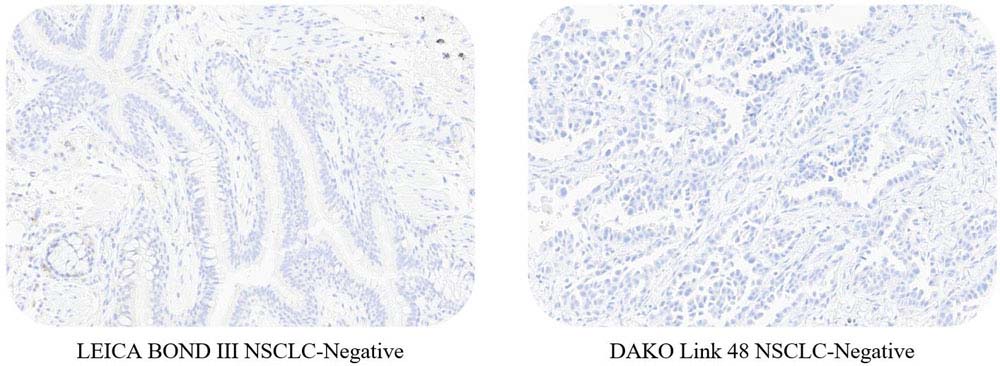
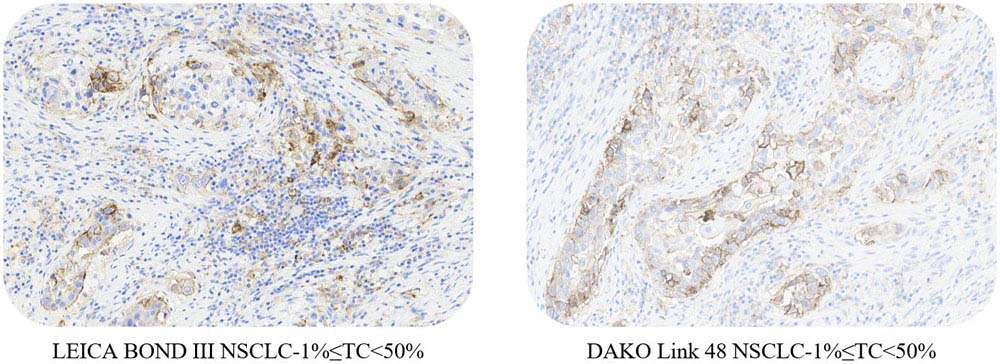
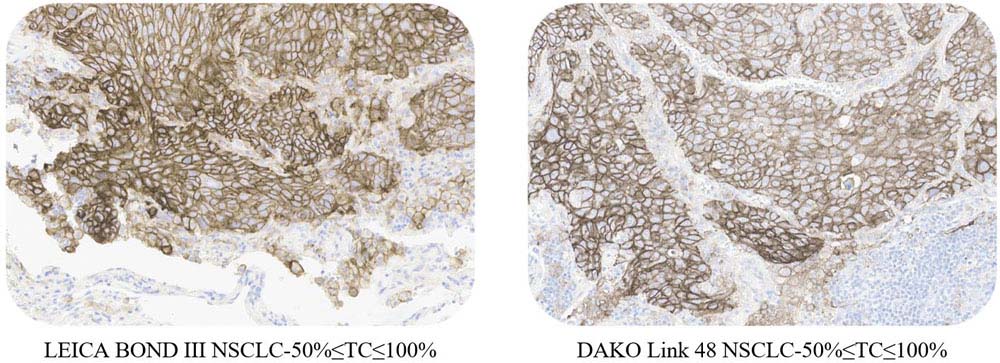
Use ACRO PD-L1 5D3 kit to stain NSCLC tissue samples at Leica BOND-III and Dako link 48.
用户评价 发表评论















