产品详情
组分(Materials Provided)
IDComponentsSizeRAS207-C01Pre-coated Anti-Influenza B (HA) Antibody Microplate1 plateRAS207-C02Influenza B/Victoria Lineage (HA) Standard20 μgRAS207-C03HRP-Anti-Influenza B (HA) Antibody20 μgRAS207-C0410×Washing Buffer50 mLRAS207-C052×Dilution Buffer50 mLRAS207-C06Substrate Solution12 mLRAS207-C07Stop Solution7 mL产品概述(Product Overview)
Influenza B Viruses Hemagglutinin (HA) Specific ELISA Kit is based on the sandwich-ELISA method and is used to specifically detect and quantitatively determine Influenza B Viruses Hemagglutinin (HA) in vaccine samples. The kit specifically identifies influenza B virus Victoria and Yamagata lineages HA protein, and is designed to provide a reliable solution for the manufacturing and quality control of HA vaccine development.
重构方法(Reconstitution)
Please see Certificate of Analysis for details of reconstitution instruction and specific concentration.
存储(Storage)
1. Unopened kit should be stored at 2℃-8℃ upon receiving.
2. Find the expiration date on the outside packaging and do not use reagents past their expiration date.
3. The opened kit should be stored per components table. The shelf life is 30 days from the date of opening.
质量管理控制体系(QMS)
数据展示
典型数据-Typical Data
Please refer to DS document for the assay protocol.
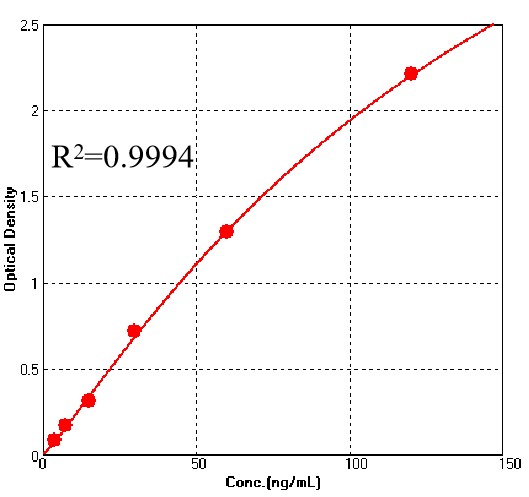
For each experiment, a standard curve needs to be set for each micro-plate, and the specific OD value may vary depending on different laboratories, testers, or equipments. The following example data is for reference only.
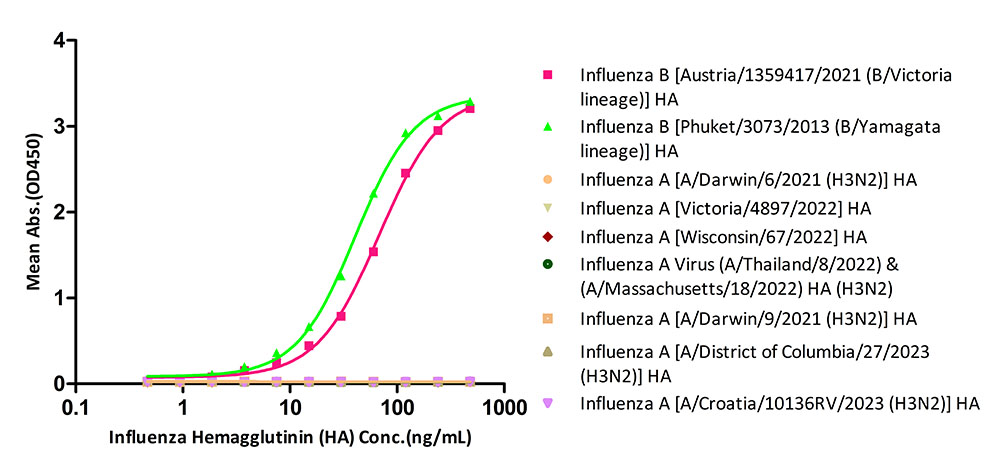
The kit has been tested to specifically identify Influenza B [Austria/1359417/2021 (B/Victoria lineage)] HA and Influenza B [Phuket/3073/2013 (B/Yamagata lineage)] HA, and do not identify Influenza A [Victoria/4897/2022 (H1N1)] HA, Influenza A [Wisconsin/67/2022(H1N1)] HA, Influenza A (A/Thailand/8/2022) & (A/Massachusetts/18/2022) HA (H3N2), Influenza A [A/Darwin/6/2021 (H3N2)] HA, Influenza A [A/Darwin/9/2021 (H3N2)] HA, Influenza A [A/District of Columbia/27/2023 (H3N2)] HA and Influenza A [A/Croatia/10136RV/2023 (H3N2)] HA.
用户评价 发表评论















