产品信息
优势特色(Features)
- Designed under ISO 9001:2015 and ISO 13485:2016
- Manufactured and QC tested under a GMP compliance factory
- Animal-Free materials
- Beta-lactam materials free
- Batch-to-batch consistency
- Stringent quality control tests
表达区间及表达系统(Source)
GMP Human GM-CSF Protein (GMP-GMFH28) is expressed from human 293 cells (HEK293). It contains AA Ala 18 - Glu 144 (Accession # P04141).
Predicted N-terminus: Ala 18
Request for sequence
蛋白结构(Molecular Characterization)

This protein carries no "tag".
The protein has a calculated MW of 14.5 kDa. The protein migrates as 25 kDa±3 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under reducing (R) condition (SDS-PAGE) due to glycosylation.
内毒素(Endotoxin)
Less than 10 EU/mg by the LAL method.
宿主蛋白残留(Host Cell Protein)
<0.5 ng/µg of protein tested by ELISA.
宿主核酸残留(Host Cell DNA)
<0.02 ng/μg of protein tested by qPCR.
无菌(Sterility)
The sterility testing was performed by membrane filtration method described in CP<1101>, USP<71> and Eur. Ph. 2.6.1.
支原体(Mycoplasma)
Negative.
纯度(Purity)
>95% as determined by SDS-PAGE.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with protectants.
Contact us for customized product form or formulation.
运输(Shipping)
This product is supplied and shipped with blue ice, please inquire the shipping cost.
存储(Storage)
Upon receipt, store it immediately at -20°C or lower for long term storage.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 5 years in lyophilized state;
- -70°C for 12 months under sterile conditions after reconstitution.
产品数据图
电泳(SDS-PAGE)
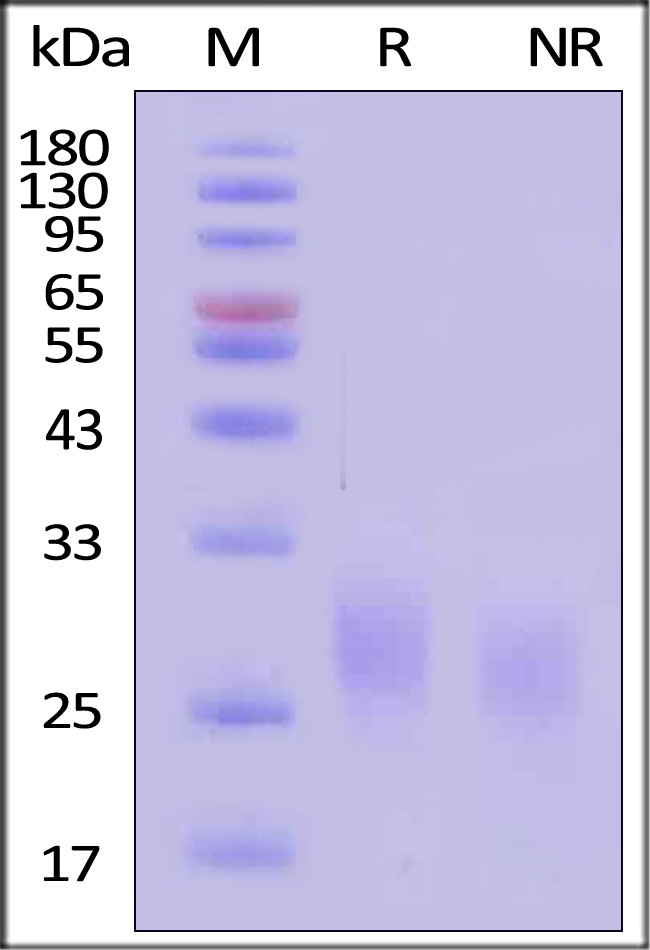
GMP Human GM-CSF Protein on SDS-PAGE under reducing (R) and non-reducing (NR) conditions. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95% (With Star Ribbon Pre-stained Protein Marker).
活性(Bioactivity)-Bioactivity CELL BASE
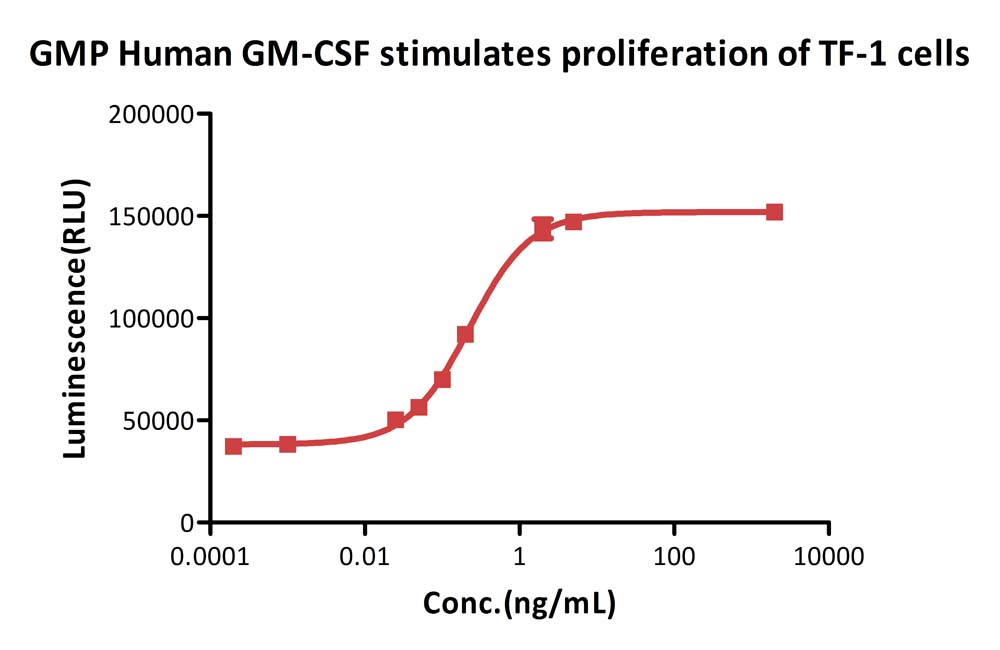
GMP Human GM-CSF Protein (Cat. No. GMP-GMFH28) stimulates proliferation of TF-1 cells. The specific activity of GMP Human GM-CSF Protein is > 5.00ⅹ10^6 IU/mg, which is calibrated against human GM-CSF WHO International Standard (NIBSC code: 88/646) (QC tested).
Protocol
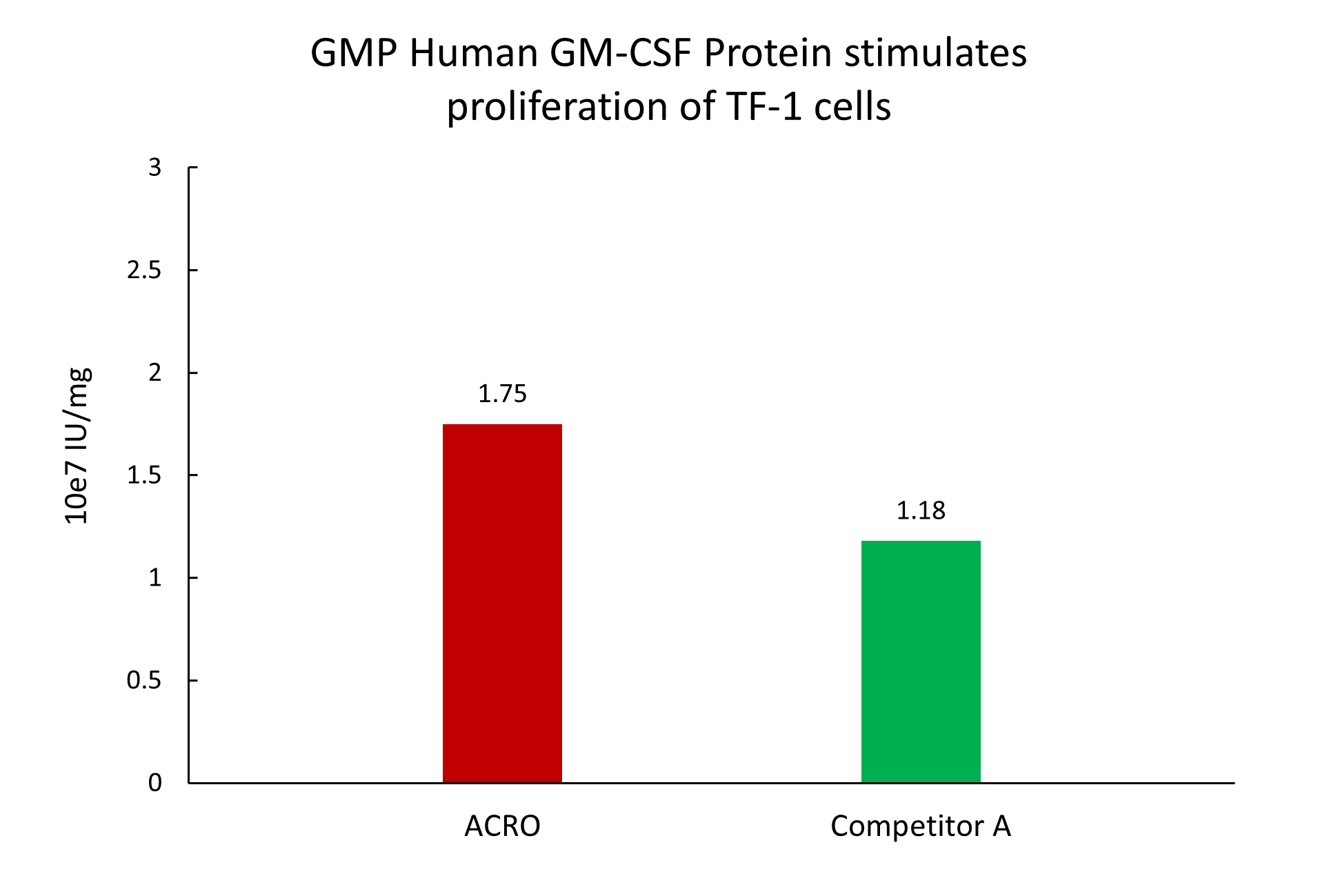
The activity of GMP Human GM-CSF Protein (Cat. No. GMP-GMFH28) was higher than other competing products.
稳定性(Stability)
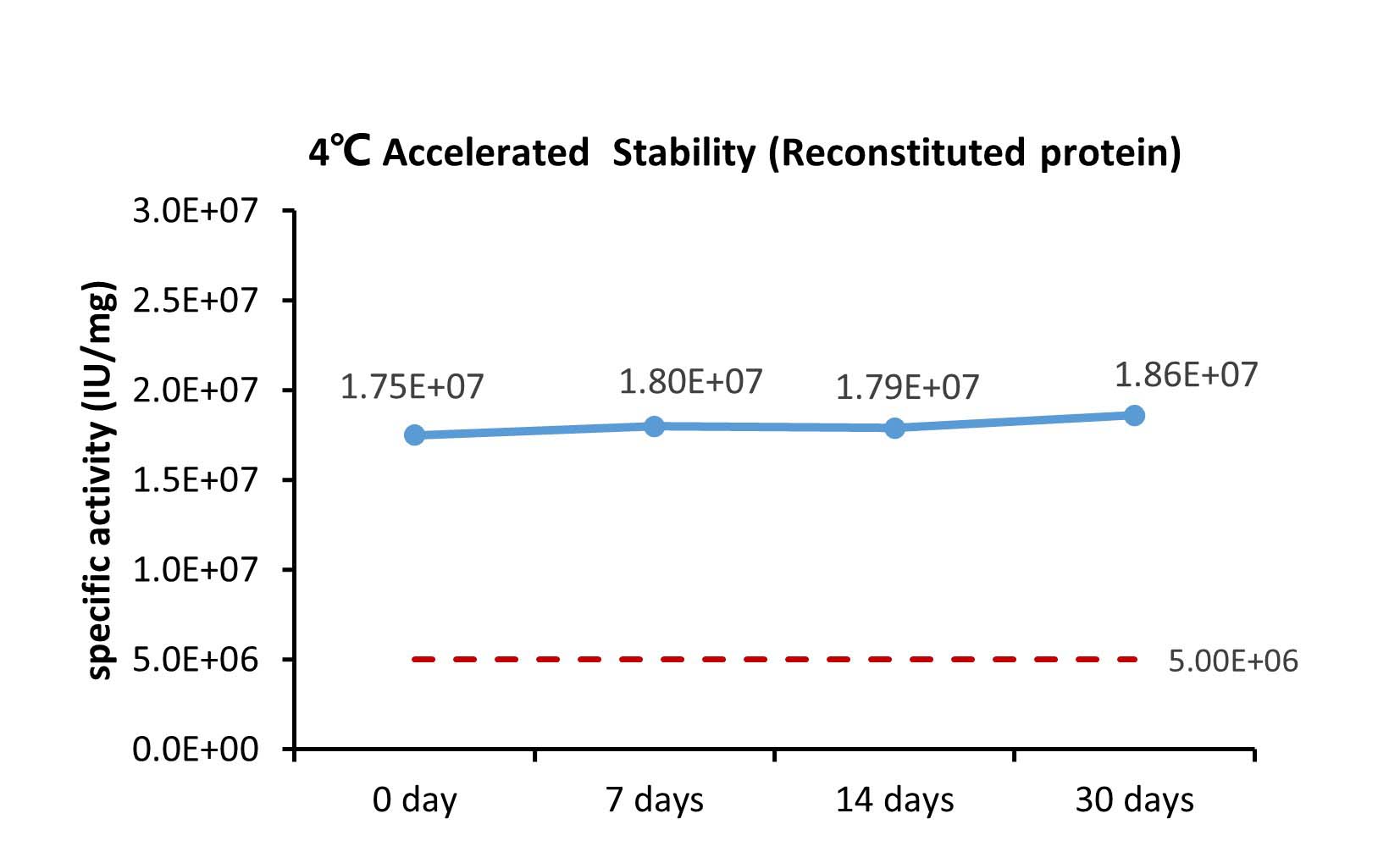
The Cell based assay shows that GMP Human GM-CSF Protein (Cat. No. GMP-GMFH28) is stable at 4°C for 180 days.
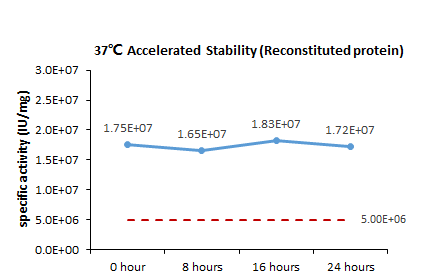
The Cell based assay shows that GMP Human GM-CSF Protein (Cat. No. GMP-GMFH28) is stable at 37℃ for 24 hours.
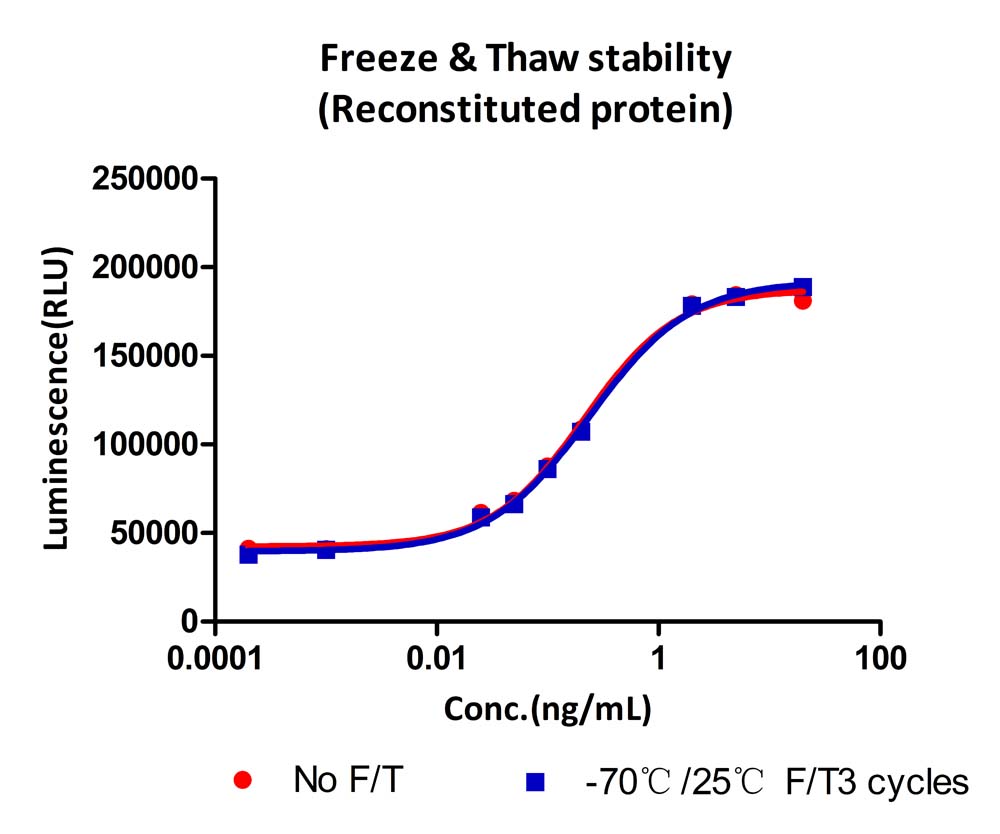
The Cell based assay shows that GMP Human GM-CSF Protein (Cat. No. GMP-GMFH28) is stable after freezing and thawing 3 times.

The Cell based assay shows batch-to-batch consistency between Acro's GMP and PG GM-CSF.
产品评论 发表评论

背景
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is also known as Colony stimulating factor 2 (granulocyte-macrophage), is a cytokine initially characterized by its ability to induce colonies of granulocytes and macrophages from myeloid progenitor cells, and is secreted by macrophages, T cells, mast cells, endothelial cells and fibroblasts. GM-CSF is a cytokine that functions as a white blood cell growth factor. GM-CSF stimulates stem cells to produce granulocytes (neutrophils, eosinophils, and basophils) and monocytes. Monocytes exitthe circulation and migrate into tissue, whereupon they mature into macrophages and dendritic cells. Thus, it is part of the immune/inflammatory cascade, by which activation of a small number of macrophages can rapidly lead to an increase in their numbers, a process crucial for fighting infection. The active form of the protein is found extracellularly as a homodimer. Human GM-CSF glycosylated in its mature form. As a part of the immune/inflammatory cascade, GM-CSF promotes Th1 biased immune response, angiogenesis, allergic inflammation, and the development of autoimmunity, and thus worthy of consideration for therapeutic target. GM-CSF has also recently been evaluated in clinical trials for its potential as a vaccine adjuvant in HIV-infected patients. The preliminary results have been promising. GM-CSF is also used as a medication to stimulate the production of white blood cells following chemotherapy.















